8 (800) 511-13-70
- Main page ›
- Catalog ›
- Colostomy bags ›
- Instruction manual
Instruction manual
"WELLSTOM" heater
According to TU 32.50.50-003-85403248-2023
Single-component non-draining calorimeter "WALLSTOM"
| Manufacturer | April Limited Liability Company |
| Legal address: | 353254, Krasnodar Territory, Seversky District, Smolenskoye Settlement, Smolenskoye Station, M. Gorky Street, 43V |
| Place of production | 1. Hubei Hendry Medical Appliance Co., Ltd (Hubei Hendry Medical Appliance Co., Ltd, China) (No.2 & No.4 Building, Yinhu Industry Park, South Xiaohan Avenue, Development District, 432000 Xiaogan City, Hubei Province, PEOPLE'S REPUBLIC OF CHINA) 2. LLC Aprel 353254, Russian Federation, Krasnodar Krai, Seversky Municipal District, Smolenskoye Rural Settlement, Stanitsa Smolenskaya, M. Gorkogo Street, 43. |
| Telephone: | 8 861-212-32-82 |
| E-mail: | torgionline@gmail.com |
SAFETY PRECAUTIONS
CIRCUMSTANCES IN WHICH THE CONSUMER SHOULD CONSULT A MEDICAL PROFESSIONAL
USED SYMBOLS
ARTICLE DESIGNATION
| КО | 1 | 2 | 3 | 4 | 5 | 6 | 7 | / | 8 |
where:
| КО | - | name of the execution option: | КО – single-component colostomy bag | ||
| 1 | - | the presence of a draining hole | 2 – non -drainable | ||
| 2 | - | type of clamp | 0 – Without clip | ||
| 3 | - | filter availability | 8 – without filter | ||
| 9 – with filter | |||||
| 4 | - | type of substrate | 1 – one-sided | ||
| 2 – two-sided | |||||
| 5 | - | bag color | 6 – bodily | ||
| 6 | - | the presence of an additional adhesive ring | 1 – there is | ||
| 0 – missing | |||||
| 7 | - | adhesive plate type | 1 – the plate is flat | ||
| 8 | - | diameter of the cut-out hole | The starting hole | maximum diameter of the cut-out hole | |
| 1060 | 10 mm | 60 mm | |||
| 1070 | 10 mm | 70 mm | |||
Name
Calorimeter "WELLSTOM" according to TU 32.50.50-003-85403248-2023
Purpose
The WELLSTOM калоприемник is used to collect intestinal contents and protect the skin from their aggressive effects.
The product is not sterile and is intended for single-use.
It is not subject to cleaning, sterilization, disinfection, maintenance, or repair.
EXECUTION OPTION
The single-component non-draining calorimeter.
APPEARANCE AND GENERAL DESCRIPTION OF THE DESIGN VARIANT

The single-component calorimeter has a built-in adhesive plate.
The stoma bag is made of opaque, multi-layered, odor-resistant brown material with a one-sided or double-sided coating of soft polymer material (substrate), with or without a carbon filter.
It does not have additional openings for emptying during operation.

The adhesive plate of the stool collector should be flexible and flat, with a hydrocolloid adhesive.
The adhesive plate must have a cut-out hole with or without a start hole. The adhesive plate may have a template for cutting holes for the stoma located on the protective coating of the plate
INDICATIONS FOR USING THE IMPLEMENTATION OPTION
The product is used to collect intestinal contents and protect the skin from their aggressive effects in case of ileal, colonic or rectal stoma.
Used at the doctor's prescription.
Contraindications
Individual intolerance to materials.
SIDE EFFECTS
When used, it may cause skin redness, rash, peeling, itching, and burning.
SCOPE OF APPLICATION
In oncology, urology, gastroenterology, dermatology, at home, and in medical and preventive institutions.
POTENTIAL CONSUMER
Stomied patients, patients' relatives, care specialists, and medical professionals.
Not recommended for self-use by young children and individuals with limited health capabilities
METHOD OF APPLICATION:

1. Rinse the stoma and the surrounding skin with warm water and dry to keep the skin clean and dry.
2. If necessary, prepare the skin around the stoma as recommended by your healthcare provider.

3. Use the measuring card to check the size of your stoma.

4. Before cutting the hole in the adhesive plate, separate the front and back films of the ostomy bag body to avoid cutting them with scissors.

5. According to the measured shape and size of the stoma, cut a hole of the appropriate size in the adhesive plate. The hole diameter is usually about 2 mm larger than the diameter of the stoma.
Note:
Do not exceed the maximum hole size according to the product marking.
At low ambient temperatures, warm the adhesive plate with your hands for a few minutes

6. Remove the protective film from the adhesive plate

7. When installing the ostomy bag, first blow some air into the bag to prevent the walls of the ostomy bag from sticking together, then align the hole cut in the adhesive plate with the ostomy, and lightly press the plate against the ostomy, then slowly press it around. The adhesive plate will stick to the skin around the ostomy

When removing the stool collector, hold the skin around the adhesive plate with one hand and slowly peel off the plate with the other hand. If the adhesive plate is stuck too tightly, do not pull it hard to avoid damaging the skin. If necessary, use warm water or a skin cleanser.
If there are gases in the bag, use a needle to puncture the air at the top of the bag.
The heat sink may have a carbon filter, which is necessary for removing gases and neutralizing odors, preventing the bag from swelling and its walls from sticking together.
Technical specifications
| Characteristic | Meaning | |
| Length (L), mm | 226,0 | |
| Width (A), mm | 152,0 | |
| Thickness, mm, max | 5,0 | |
| Capacity, ml | 400± 30 | |
| Diameter of the cut-out hole of the adhesive plate for part (8) of the article | Starting hole (b), mm | Maximum diameter of the cut hole (D), mm |
| 1060 | 10 mm | 60 mm |
| 1070 | 10 mm | 70 mm |
Structure
Activated carbon, hydrocolloid (hot-melt adhesive, polyisobutylene, potato starch, carboxymethylcellulose, plasticizer, and antioxidant), polyvinyl chloride (PVC), and polyethylene (PET).
CONDITIONS OF TRANSPORTATION, STORAGE, AND OPERATION
Temperature 0 – 40℃;
Relative humidity: not more than 80%.
The product should be protected from heavy pressure, direct sunlight, rain and snow.
DISPOSAL OF A MEDICAL PRODUCT
The used product should be disposed of. The non-draining stool bag is not equipped with a drainage hole and is not suitable for removing the contents of the stoma bag before disposal. The used product should not be disposed of in the sewer.
Disposal at home:
After use, dispose of it with household solid waste.
Disposal in medical and preventive institutions
Unused products and packaging are disposed of in accordance with local regulations as Class A waste.
When using medical devices in infectious disease departments, used devices are disposed of in accordance with local regulations as Class B waste.
Expiration date
Product shelf life/storage period: 3 years from the date of manufacture indicated on the packaging.
Do not use the product after the expiration date
MANUFACTURER'S GUARANTEES
The manufacturer guarantees that the product meets the declared specifications if the consumer follows the operating, transportation, and storage conditions.
The product has a 3-year warranty period from the date of manufacture indicated on the packaging.
INFORMATION ABOUT COMPLAINTS
Claims are made in accordance with the established procedure at the manufacturer's address
APRIL LLC, Russia
"WELLSTOM" heater
according to TU 32.50.50-003-85403248-2023
Single-component drainable calorimeter "WELLSTOM"
| Manufacturer | April Limited Liability Company |
| Legal address: | 353254, Krasnodar Territory, Seversky District, Smolenskoye Settlement, Smolenskoye Station, M. Gorky Street, 43V |
| Place of production | 1. Hubei Hendry Medical Appliance Co., Ltd (Hubei Hendry Medical Appliance Co., Ltd, China) (No.2 & No.4 Building, Yinhu Industry Park, South Xiaohan Avenue, Development District, 432000 Xiaogan City, Hubei Province, PEOPLE'S REPUBLIC OF CHINA) 2. LLC Aprel 353254, Russian Federation, Krasnodar Krai, Seversky Municipal District, Smolenskoye Rural Settlement, Stanitsa Smolenskaya, M. Gorkogo Street, 43. |
| Telephone: | 8 861-212-32-82 |
| E-mail: | torgionline@gmail.com |
SAFETY PRECAUTIONS
CIRCUMSTANCES IN WHICH THE CONSUMER SHOULD CONSULT A MEDICAL PROFESSIONAL
USED SYMBOLS
ARTICLE DESIGNATION
| КО | 1 | 2 | 3 | 4 | 5 | 6 | 7 | / | 8 |
where:
| КО | - | name of the execution option: | КО – single-component colostomy bag | ||
| 1 | - | the presence of a draining hole | 1 –drainable | ||
| 2 | - | type of clamp | 1 – The clamp is flexible | ||
| 2 – Snap-on clip | |||||
| 3 – Velcro closure | |||||
| 4 – Velcro with petals | |||||
| 3 | - | filter availability | 8 – without a filter | ||
| 9 – with a filter | |||||
| 4 | - | type of substrate | 1 – one-sided | ||
| 2 – two-sided | |||||
| 5 | - | bag color | 6 – bodily | ||
| 5 – transparent | |||||
| 7 – gray | |||||
| 6 | - | the presence of an additional adhesive ring | 1 – there is | ||
| 0 – absent | |||||
| 7 | - | adhesive plate type | 1 – the plate is flat | ||
| 2 – the plate is convex | |||||
| 8 | - | diameter of the cut-out hole | The starting hole | maximum diameter of the cut-out hole | |
| 1010 | 10 mm | 100 mm | |||
| 1035 | 10 mm | 35 mm | |||
| 1040 | 10 mm | 40 mm | |||
| 1060 | 10 mm | 60 mm | |||
| 1065 | 10 mm | 65 mm | |||
| 1070 | 10 mm | 70 mm | |||
| 1075 | 10 mm | 75 mm | |||
| 1080 | 10 mm | 80 mm | |||
| 1085 | 10 mm | 85 mm | |||
| 1510 | 15 mm | 100 mm | |||
| 1538 | 15 mm | 38 mm | |||
| 1545 | 15 mm | 45 mm | |||
| 1557 | 15 mm | 57 mm | |||
| 1576 | 15 mm | 76 mm | |||
| 1580 | 15 mm | 80 mm | |||
Name
"WALLSTOM" Calorimeter according to the Technical Specifications 32.50.50-003-85403248-2023
Purpose
The WELLSTOM stool collector is used to collect intestinal contents and protect the skin from their aggressive effects.
The product is not sterile and is intended for single-use.
It is not subject to sterilization, disinfection, maintenance, or repair.
EXECUTION OPTION
The calorimeter is single-component and drains.
APPEARANCE AND GENERAL DESCRIPTION OF THE DESIGN VARIANT

The single-component stool bag has a built-in adhesive plate.
The stoma bag is made of opaque or transparent, multi-layered, odor-resistant material in white, brown, yellow, or gray, with a single-sided or double-sided coating of soft polymer material (substrate), with or without a carbon filter.
It has a closing opening for emptying during use. It has a built-in clamp for the drained opening or is equipped with a reusable clamp.

The adhesive plate of the stool collector must be flexible, round or oval, flat or convex, with a hydrocolloid adhesive.
The adhesive plate must have a cut-out hole with or without a starting hole. The adhesive plate may have a template for cutting holes for the stoma, located on the protective coating of the plate
The calorimeter may have a built-in clamp for the drained hole or be equipped with a reusable clamp

Built-in clip
Velcro-release

Reusable clip

Flexible clamp
INDICATIONS FOR USING THE IMPLEMENTATION OPTION
The product is used to collect intestinal contents and protect the skin from their aggressive effects in case of ileal, colonic or rectal stoma.
Used at the doctor's prescription.
Contraindications
Individual intolerance to materials.
SIDE EFFECTS
When used, it may cause skin redness, rash, peeling, itching, and burning.
SCOPE OF APPLICATION
In oncology, urology, gastroenterology, dermatology, at home, and in medical and preventive institutions.
POTENTIAL CONSUMER
Stomized patients, patients' relatives, care specialists, and medical professionals.
Not recommended for self-use by young children and individuals with limited health capabilities
METHOD OF APPLICATION:

1. Rinse the stoma and the surrounding skin with warm water and dry to keep the skin clean and dry.
2. If necessary, prepare the skin around the stoma according to the doctor's instructions.

3. Use a measuring chart to check the size of your stoma.

4. Before cutting a hole in the adhesive plate, separate the front and back films of the ostomy bag body to avoid cutting them with scissors.

5. According to the measured shape and size of the stoma, cut a suitable-sized hole in the adhesive plate. The hole diameter is usually about 2 mm larger than the diameter of the stoma.
Note:
Do not exceed the maximum hole size according to the product marking.
If the ambient temperature is low, warm the adhesive plate with your hands for a few minutes.

6. Remove the protective film from the adhesive plate

7. When installing the ostomy bag, first blow some air into the bag to prevent the walls of the ostomy bag from sticking together, then align the hole cut in the adhesive plate with the ostomy, and gently press the plate against the ostomy, then slowly press it around. The adhesive plate will stick to the skin around the ostomy.
Install the bag clip.
8. Install a flexible clamp or a snap clamp 2-3 cm from the edge of the drained hole, or attach the built-in Velcro clamp.

Snap-on clip

Velcro

Flexible clamp

To empty the drained stool container, remove the drainage hole clamp.
Snap-on clamp: Use your finger to press the locking tab of the snap-on clamp and fully open the clamp.
Velcro: Unfasten the Velcro auxiliary tabs, then unfasten the main Velcro tab and unfold the drained stool container.
Flexible clamp: Unfold the flexible clamp from both sides and unfold the drained stool container.

1. Open the opening and remove the contents of the ostomy bag, rinse the drainage opening, and dry it with a paper towel. Align the tail, clamp the sealing clip, and continue using it.

2. Align the drainage hole, tighten the sealing clip, and continue using the caliper.

When removing the stool collector, hold the skin around the adhesive plate with one hand and slowly peel off the plate with the other hand. If the adhesive plate is stuck too tightly, do not pull it hard to avoid damaging the skin. If necessary, use warm water or a skin cleanser.
The heat sink may have a carbon filter, which is necessary for removing gases and neutralizing odors, preventing the bag from swelling and its walls from sticking together.
Technical specifications
| Characteristic | Meaning | |
| Length (L), mm | 293,0 | |
| Width (A), mm | 152,0 | |
| Thickness, mm, max | 5.0 | |
| Capacity, ml | 400± 30 | |
| Width of the drainage hole, mm | 70 | |
| Diameter of the cut-out hole of the adhesive plate for part (8) of the article | Starting hole (b), mm | Maximum diameter of the cut hole (D), mm |
| 1010 | 10 mm | 100 mm |
| 1035 | 10 mm | 35 mm |
| 1040 | 10 mm | 40 mm |
| 1060 | 10 mm | 60 mm |
| 1065 | 10 mm | 65 mm |
| 1070 | 10 mm | 70 mm |
| 1080 | 10 mm | 75 mm |
| 1085 | 10 mm | 80 mm |
| 1510 | 15 mm | 85 mm |
| 1538 | 15 mm | 100 mm |
| 1545 | 15 mm | 38 mm |
| 1557 | 15 mm | 45 mm |
| 1576 | 15 mm | 57 mm |
| 1580 | 15 mm | 76 mm |
Structure
Activated carbon, hydrocolloid (hot-melt adhesive, polyisobutylene, potato starch, carboxymethylcellulose, plasticizer, and antioxidant), medical adhesive tape, polyvinyl chloride (PVC), and polyethylene (PET).
CONDITIONS OF TRANSPORTATION, STORAGE, AND OPERATION
Temperature 0 – 40℃;
Relative humidity: not higher than 80%.
The product should be protected from strong pressure, direct sunlight, rain and snow.
DISPOSAL OF A MEDICAL PRODUCT
At home:
after use, dispose of it with solid household waste.
In medical and preventive institutions:
Unused products and packaging are disposed of in accordance with local regulations as Class A waste.
When using medical products in infectious disease departments, used products are disposed of in accordance with local regulations as Class B waste.
Expiration date
Product shelf life: 3 years from the date of manufacture indicated on the packaging.
Do not use the product after the expiration date.
MANUFACTURER'S GUARANTEES
The manufacturer guarantees that the product meets the declared specifications if the user follows the operating, transportation, and storage conditions.
The product has a 3-year warranty period from the date of manufacture indicated on the packaging.
INFORMATION ABOUT COMPLAINTS
Claims are made in accordance with the established procedure at the manufacturer's address
APRIL LLC, Russia
Two-component non-draining calorimeter
| Manufacturer | April Limited Liability Company |
| Legal address: | 353254, Krasnodar Territory, Seversky District, Smolenskoye Settlement, Smolenskoye Station, M. Gorky Street, 43V |
| Place of production | 1. Hubei Hendry Medical Appliance Co., Ltd (Hubei Hendry Medical Appliance Co., Ltd, China) (No.2 & No.4 Building, Yinhu Industry Park, South Xiaohan Avenue, Development District, 432000 Xiaogan City, Hubei Province, PEOPLE'S REPUBLIC OF CHINA) 2. LLC Aprel 353254, Russian Federation, Krasnodar Krai, Seversky Municipal District, Smolenskoye Rural Settlement, Stanitsa Smolenskaya, M. Gorkogo Street, 43. |
| Telephone: | 8 861-212-32-82 |
| E-mail: | torgionline@gmail.com |
SAFETY PRECAUTIONS
CIRCUMSTANCES IN WHICH THE CONSUMER SHOULD CONSULT A MEDICAL PROFESSIONAL
USED SYMBOLS
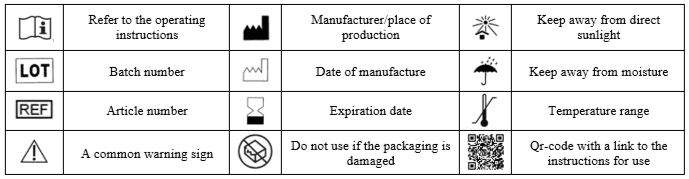
ARTICLE DESIGNATION
| МД | 1 | 2 | 3 | 4 | 5 | 6 | 7 | / | 8 |
где:
| МД | - | name of the execution option: | МД – two-component colostomy bag |
| 1 | - | the presence of a draining hole | 2 – untrained |
| 2 | - | type of clamp | 0 – Without clip |
| 3 | - | filter availability | 8 – without a filter |
| 9 – with a filter | |||
| 4 | - | type of substrate | 1 – one-sided |
| 2 – two-sided | |||
| 5 | - | bag color | 6 – bodily |
| 6 | - | belt fastening rings | 1 – there is |
| 0 – absent | |||
| 7 | - | flange diameter | 50 - 50 mm |
| 60 - 60 mm | |||
| 80 - 80 mm |
for adhesive plate:
| 1 | 2 | / | 3 | 4 |
where:
| 1 | - | name of the execution option: | П1 – the plate is flat | ||
| П2 – the plate is convex | |||||
| П3 – the plate is oval | |||||
| 2 | - | belt fastening rings | 1 – there is | ||
| 0 – absent | |||||
| 3 | - | flange diameter | 50 – 50 mm | ||
| 60 – 60 mm | |||||
| 80 – 80 mm | |||||
| 4 | - | diameter of the cut-out hole | The starting hole | maximum diameter of the cut-out hole | |
| 1045 | 10 mm | 45 mm | |||
| 1050 | 50 mm | ||||
| 1055 | 10 mm | 55 mm | |||
| 1060 | 10 mm | 60 mm | |||
| 1075 | 10 mm | 75 mm | |||
| 1080 | 10 mm | 80 mm | |||
| 1545 | 15 mm | 45 mm | |||
Name
"WALLSTOM" Calorimeter according to the Technical Specifications 32.50.50-003-85403248-2023
Purpose
The WELLSTOM stool collector is used to collect intestinal contents and protect the skin from their aggressive effects.
The product is not sterile and is intended for single-use.
It is not subject to sterilization, disinfection, maintenance, or repair.
EXECUTION OPTION
Two-component non-draining calorimeter
APPEARANCE AND GENERAL DESCRIPTION OF THE DESIGN VARIANT

A two-component stool receiver consists of two interconnected parts with a flange connection: an adhesive plate and a stoma bag.
The stoma bag is made of an opaque, multi-layered, odor-resistant material that is either white or brown in color, with a one-sided or double-sided coating of soft polymer material (substrate), and may or may not have a carbon filter.
The stoma bag of a two-component stool receiver may have belt attachments on the flange part.

The adhesive plate of the stool collector must be flexible, round or oval, flat or convex, with a hydrocolloid adhesive.
The adhesive plate must have a cut-out hole with or without a starting hole. The adhesive plate may have a template for cutting holes for the stoma, located on the protective coating of the plate.
The adhesive plate of a two-component stool collector may have belt attachments on the flange part.
INDICATIONS FOR USING THE IMPLEMENTATION OPTION
The product is used to collect intestinal contents and protect the skin from their aggressive effects in case of ileal, colonic or rectal stoma.
Used at the doctor's prescription.
Contraindications
Individual intolerance to materials.
SIDE EFFECTS
When used, it may cause skin redness, rash, peeling, itching, and burning.
SCOPE OF APPLICATION
In oncology, urology, gastroenterology, dermatology, at home, and in medical and preventive institutions.
POTENTIAL CONSUMER
Stomized patients, patients' relatives, care specialists, and medical professionals.
Not recommended for self-use by young children and individuals with limited health capabilities
METHOD OF APPLICATION:

1. Rinse the stoma and the surrounding skin with warm water and dry to keep the skin clean and dry.
2. If necessary, prepare the skin around the stoma according to the doctor's instructions.

3. Use a measuring chart to check the size of your stoma.

4. According to the measured shape and size of the stoma, cut a suitable-sized hole in the adhesive plate. The hole diameter is usually about 2 mm larger than the diameter of the stoma.
Note:
Do not exceed the maximum hole size according to the product label.
If the ambient temperature is low, warm the adhesive plate with your hands for a few minutes.

5. Remove the protective film from the adhesive plate

6. Align the hole cut in the adhesive plate with the stoma and press the plate lightly against the stoma, then slowly press it around the stoma. The adhesive plate will stick to the skin around the stoma.
When using the belt:
Wrap the belt around your body and adjust it to the desired length, then insert the fasteners on the belt into the special holes on the stool bag or the adhesive plate.

7. When installing the ostomy bag, first blow some air into the bag to prevent the walls of the ostomy bag from sticking together. Align the flange of the ostomy bag with the flange of the adhesive plate and gradually press down from the top. Once the entire flange is secured, gently pull on the ostomy bag to check the tightness of the attachment.

When the ostomy bag is filled one-third or one-half full, replace the ostomy bag.
Grasp the tabs on the adhesive plate flange, separate the ostomy bag flange from the top to the bottom, and slowly remove the ostomy bag. Install a new ostomy bag according to step 7.
Note:
The adhesive plate of the two-piece ostomy bag can remain attached for 3 to 5 days. Longer use may cause skin irritation or leakage.

When removing the adhesive plate, hold the skin around it with one hand and slowly peel off the plate with the other hand. If the adhesive plate is stuck too tightly, do not pull it hard to avoid damaging the skin. If necessary, use warm water or a skin cleanser.
The heat sink may have a carbon filter, which is necessary for removing gases and neutralizing odors, preventing the bag from swelling and its walls from sticking together.
Technical specifications
| Characteristic | Meaning | |
| Two-component non-draining stool bag | ||
| Length (L), mm | 226,0 | |
| Width (A), mm | 152,0 | |
| Thickness, mm, max | 6,0 | |
| Capacity, ml | 400± 30 | |
| Flange diameter (D), mm for part (7) of the article | ||
| 50 | 50 mm | |
| 60 | 60 mm | |
| 80 | 80 mm | |
| Adhesive plate | ||
| Flange diameter (d), mm for part (3) of the article | ||
| 50 | 50 mm | |
| 60 | 60 mm | |
| 80 | 80 mm | |
| Diameter of the cut-out hole of the adhesive plate for part (4) of the article | ||
| 1045 | 10 mm | 45 mm |
| 1050 | 10 mm | 50 mm |
| 1055 | 10 mm | 55 mm |
| 1060 | 10 mm | 60 mm |
| 1080 | 10 mm | 80 mm |
| 1538 | 15 mm | 38 mm |
| 1545 | 15 mm | 45 mm |
| Convex plate height (h), mm | 4 | |
| Belt length range, cm, no more than | 52 – 100 (±10) | |
| Belt width, cm3 | 3 | |
Structure
ABS plastic, activated carbon, hydrocolloid (hot-melt adhesive, polyisobutylene, potato starch, carboxymethylcellulose, plasticizer, antioxidant), polyvinyl chloride (PVC), polyester, polyethylene (PET), thermoplastic polyurethane
CONDITIONS OF TRANSPORTATION, STORAGE, AND OPERATION
Temperature 0 – 40℃;
Relative humidity: not higher than 80%.
The product should be protected from strong pressure, direct sunlight, rain and snow.
DISPOSAL OF A MEDICAL PRODUCT
At home:
after use, dispose of it with solid household waste.
In medical and preventive institutions:
Unused products and packaging are disposed of in accordance with local regulations as Class A waste.
When using medical products in infectious disease departments, used products are disposed of in accordance with local regulations as Class B waste.
Expiration date
Product shelf life: 3 years from the date of manufacture indicated on the packaging.
Do not use the product after the expiration date.
MANUFACTURER'S GUARANTEES
The manufacturer guarantees that the product meets the declared specifications if the user follows the operating, transportation, and storage conditions.
The product has a 3-year warranty period from the date of manufacture indicated on the packaging.
INFORMATION ABOUT COMPLAINTS
Claims are made in accordance with the established procedure at the manufacturer's address
APRIL LLC, Russia
Two-component drainable calorimeter
| Manufacturer | April Limited Liability Company |
| Legal address: | 353254, Krasnodar Territory, Seversky District, Smolenskoye Settlement, Smolenskoye Station, M. Gorky Street, 43V |
| Place of production | 1. Hubei Hendry Medical Appliance Co., Ltd (Hubei Hendry Medical Appliance Co., Ltd, China) (No.2 & No.4 Building, Yinhu Industry Park, South Xiaohan Avenue, Development District, 432000 Xiaogan City, Hubei Province, PEOPLE'S REPUBLIC OF CHINA) 2. LLC Aprel 353254, Russian Federation, Krasnodar Krai, Seversky Municipal District, Smolenskoye Rural Settlement, Stanitsa Smolenskaya, M. Gorkogo Street, 43. |
| Telephone: | 8 861-212-32-82 |
| E-mail: | torgionline@gmail.com |
SAFETY PRECAUTIONS
CIRCUMSTANCES IN WHICH THE CONSUMER SHOULD CONSULT A MEDICAL PROFESSIONAL
USED SYMBOLS

ARTICLE DESIGNATION
| МД | 1 | 2 | 3 | 4 | 5 | 6 | / | 7 |
где:
| МД | - | name of the execution option: | МД – two-component colostomy bag |
| 1 | - | the presence of a draining hole | 2 – untrained |
| 1 – drainable | |||
| 2 | - | type of clamp | 1 – The clamp is flexible |
| 2 – Snap-on clip | |||
| 3 – Velcro closure | |||
| 4 – Velcro with petals | |||
| 3 | - | filter availability | 8 – without a filter |
| 9 – with a filter | |||
| 4 | - | type of substrate | 1 – one-sided |
| 2 – two-sided | |||
| 5 | - | bag color | 6 – bodily |
| 7 – gray | |||
| 6 | - | belt fastening rings | 1 – there is |
| 0 – absent | |||
| 7 | - | flange diameter | 50 - 50 mm |
| 60 - 60 mm | |||
| 80 - 80 mm |
for adhesive plate:
| 1 | 2 | / | 3 | 4 |
где:
| 1 | - | name of the execution option: | П1 – the plate is flat | ||
| П2 – the plate is convex | |||||
| П3 – the plate is oval | |||||
| 2 | - | belt fastening rings | 1 – there is | ||
| 0 – absent | |||||
| 3 | - | flange diameter | 50 – 50 mm | ||
| 60 – 60 mm | |||||
| 80 – 80 mm | |||||
| 4 | - | diameter of the cut-out hole | The starting hole | maximum diameter of the cut-out hole | |
| 1045 | 10 mm | 45 mm | |||
| 1050 | 50 mm | ||||
| 1055 | 10 mm | 55 mm | |||
| 1060 | 10 mm | 60 mm | |||
| 1075 | 10 mm | 75 mm | |||
| 1080 | 10 mm | 80 mm | |||
| 1538 | 15 mm | 38 mm | |||
| 1545 | 15 mm | 45 mm | |||
Name
"WALLSTOM" Calorimeter according to the Technical Specifications32.50.50-003-85403248-2023
Purpose
"WALLSTOM" Calorimeter according to the Technical Specifications32.50.50-003-85403248-2023
EXECUTION OPTION
The calorimeter is two-component and drains.
APPEARANCE AND GENERAL DESCRIPTION OF THE DESIGN VARIANT

A two-component stool receiver consists of two flanged-connected parts – an adhesive plate and a stoma bag.
The stoma bag is produced from an opaque or transparent, multi-layered, odor-permeable material of white, brown, yellow or grey colour, with a one-sided or two-sided coating of soft polymer material (substrate), with or without a carbon filter.
It has a closing opening for emptying during operation. It has a built-in clamp of the drained opening or is equipped with a reusable clamp
The two-component stool bag may have belt attachments on the flange.

The adhesive plate of the ostomy bag should be flexible, round or oval, flat or convex, with a hydrocolloid adhesive.
The adhesive plate should have a cut-out hole with or without a starting hole. The adhesive plate may have a template for cutting holes for the ostomy located on the protective coating of the plate.
The adhesive plate of a two-component ostomy bag may have belt attachments on the flange.
The calorimeter may have a built-in clamp for the drained hole or be equipped with a reusable clamp

Built-in clip
Velcro-release

Reusable clip

Flexible clamp
INDICATIONS FOR USING THE IMPLEMENTATION OPTION
The product is used to collect intestinal contents and protect the skin from their aggressive effects in case of ileal, colonic or rectal stoma.
Used at the doctor's prescription.
Contraindications
Individual intolerance to materials.
SIDE EFFECTS
When used, it may cause skin redness, rash, peeling, itching, and burning.
SCOPE OF APPLICATION
In oncology, urology, gastroenterology, dermatology, at home, and in medical and preventive institutions.
POTENTIAL CONSUMER
Stomized patients, patients' relatives, care specialists, and medical professionals.
Not recommended for self-use by young children and individuals with limited health capabilities
METHOD OF APPLICATION:

1. Rinse the stoma and the surrounding skin with warm water and dry to keep the skin clean and dry.
2. If necessary, prepare the skin around the stoma according to the doctor's instructions.

3. Use a measuring chart to check the size of your stoma.

4. According to the measured shape and size of the stoma, cut a suitable-sized hole in the adhesive plate. The hole diameter is usually about 2 mm larger than the diameter of the stoma.
Note:
Do not exceed the maximum hole size according to the product label.
If the ambient temperature is low, warm the adhesive plate with your hands for a few minutes.

5. Remove the protective film from the adhesive plate

6. Align the hole cut in the adhesive plate with the stoma and press the plate lightly against the stoma, then slowly press it around the stoma. The adhesive plate will stick to the skin around the stoma.
When using the belt:
Wrap the belt around your body and adjust it to the desired length, then insert the fasteners on the belt into the special holes on the stool bag or the adhesive plate.

7. When installing the ostomy bag, first blow some air into the bag to prevent the walls of the ostomy bag from sticking together. Align the flange of the ostomy bag with the flange of the adhesive plate and gradually press down from the top. Once the entire flange is secured, gently pull on the ostomy bag to check the tightness of the attachment.
When using a belt:
Wrap the belt around your body and adjust it to the desired length, then insert the fasteners on the belt into the designated holes on the ostomy bag or the adhesive plate.
8. Install a flexible clamp or a snap clamp 2-3 cm from the edge of the drained hole, or attach the built-in Velcro clamp.

Snap-on clip

Velcro

Flexible clamp

To empty the drained stool container, remove the drainage hole clamp.
Snap-on clamp: Use your finger to press the locking tab of the snap-on clamp and fully open the clamp.
Velcro: Unfasten the Velcro auxiliary tabs, then unfasten the main Velcro tab and unfold the drained stool container.
Flexible clamp: Unfold the flexible clamp from both sides and unfold the drained stool container.

1. Open the opening and remove the contents of the stoma bag, rinse the drainage opening, and dry it with a paper towel. Align the tail, clamp the sealing clip, and continue using

2. Align the drainage hole, tighten the sealing clip, and continue using the caliper.

3. If necessary, rinse the ostomy bag and disconnect the flange connection from the adhesive plate.
Grip the tabs on the adhesive plate flange, separate the ostomy bag flange from the top to the bottom, and slowly remove the ostomy bag

4. Insert the washing bottle into the body of the stoma bag for cleaning, clean and dry, and install it on the adhesive plate according to step 7.
Note:
The adhesive plate of the two-component stool collector can remain stuck for 3 to 5 days. Longer use may cause skin irritation or leakage.
The heat sink may have a carbon filter, which is necessary for removing gases and neutralizing odors, preventing the bag from swelling and its walls from sticking together.
Technical specifications
| Characteristic | Meaning | |
| Two-component non-draining stool bag | ||
| Length (L), mm | 226,0 | |
| Width (A), mm | 152,0 | |
| Thickness, mm, max | 6,0 | |
| Capacity, ml | 400± 30 | |
| Flange diameter (D), mm for part (7) of the article | ||
| 50 | 50 mm | |
| 60 | 60 mm | |
| 80 | 80 mm | |
| Two-component drainable stool bag | ||
| Length (L), mm | 293,0 | |
| Width (A), mm | 152,0 | |
| Thickness, mm | 6,2 | |
| Capacity, ml | 400± 30 | |
| Width of the drainage hole, mm | 70 | |
| Flange diameter (D), mm for part (7) of the article | ||
| 50 | 50 mm | |
| 60 | 60 mm | |
| 80 | 80 mm | |
| Adhesive plate | ||
| Flange diameter (d), mm for part (3) of the article | ||
| 50 | 50 mm | |
| 60 | 60 mm | |
| 80 | 80 mm | |
| Diameter of the cut-out hole of the adhesive plate for part (4) of the article | ||
| 1045 | 10 mm | 45 mm |
| 1050 | 10 mm | 50 mm |
| 1055 | 10 mm | 55 mm |
| 1060 | 10 mm | 60 mm |
| 1080 | 10 mm | 80 mm |
| 1538 | 15 mm | 38 mm |
| 1545 | 15 mm | 45 mm |
| Convex plate height (h), mm | 4 | |
| Belt length range, cm, no more than | 52 – 100 (±10) | |
| Belt width, cm3 | 3 | |
Structure
ABS plastic, Activated carbon, hydrocolloid (hot-melt adhesive, polyisobutylene, potato starch, carboxymethylcellulose, plasticizer, antioxidant), medical adhesive tape, polyvinyl chloride (PVC), polyester, polyethylene (PET), thermoplastic polyurethane
CONDITIONS OF TRANSPORTATION, STORAGE, AND OPERATION
Temperature 0 – 40℃;
Relative humidity: not higher than 80%.
The product should be protected from strong pressure, direct sunlight, rain and snow.
DISPOSAL OF A MEDICAL PRODUCT
At home:
after use, dispose of it with solid household waste.
In medical and preventive institutions:
Unused products and packaging are disposed of in accordance with local regulations as Class A waste.
When using medical products in infectious disease departments, used products are disposed of in accordance with local regulations as Class B waste.
Expiration date
Product shelf life: 3 years from the date of manufacture indicated on the packaging.
Do not use the product after the expiration date.
MANUFACTURER'S GUARANTEES
The manufacturer guarantees that the product meets the declared specifications if the user follows the operating, transportation, and storage conditions.
The product has a 3-year warranty period from the date of manufacture indicated on the packaging.
INFORMATION ABOUT COMPLAINTS
Claims are made in accordance with the established procedure at the manufacturer's address
APRIL LLC, Russia
Our contacts
Address of the trading house:
Krasnodar Territory, Krasnodar, Morskaya Street, 15
Production address:







