8 (800) 511-13-70
- Main page ›
- Catalog ›
- Urine collectors ›
- Instruction manual
Instruction manual
Medical urinary catheter according to TU 32.50.50-004-85403248-2023
| Manufacturer | April Limited Liability Company |
| Legal address | 353254, Krasnodar Territory, Seversky District, Smolenskoye Settlement, Smolenskoye Station, M. Gorky Street, 43V |
| Place of production | 353254, Russian Federation, Krasnodar Territory, Seversky Municipal District, Smolenskoye Rural Settlement, Stanitsa Smolenskoye, M. Gorky Street, 43 |
| Telephone: | 8 861-212-32-82 |
| E-mail: | torgionline@gmail.com |
SAFETY PRECAUTIONS
| Attention! |
| For one-time use only. |
| Use it immediately after opening the package. |
| Do not use more than the recommended time limit. |
| A medical professional works with gloves. |
USED SYMBOLS

Refer to the operating instructions

Attention!
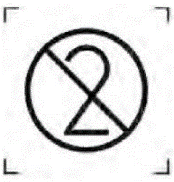
Prohibition of repeated use
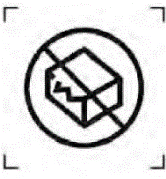
Do not use if the packaging is damaged (for individually packaged products)

Not sterile

Warning
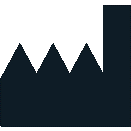
Production
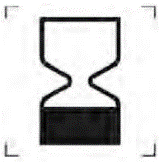
Use up to
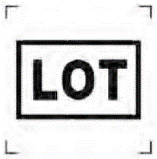
Batch Code
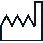
Date of manufacture
Article number for urinators:

where:
Name
Medical urinary catheter according to TU 32.50.50-004-85403248-2023
Purpose
The medical urine collector is used to collect urine during catheterization or for continuous urine collection in people who temporarily or permanently cannot perform normal urination, including bedridden patients. The product is non-sterile and single-use.
MODELS:
1. Medical foot urine collector (NC: 156390), consisting of:
1.1. Medical foot urination device for adults (article number: MN755/1, MN759/1, MN755N/1, MN759N/1, MN755/2, MN759/2, MN755N/2, MN759N/2, MN755/1, MN759N/1, MN755N/1, MN759N/1, MN755/2, MPR759/2, MPR755N/2, MPR759N/2): with a tube length of 50 cm, 90 cm; volume of 750 ml; with or without a non–woven backing - no more than 200 pcs./pack.;
1.2. Instructions for use No. WS-004-IU – 1 copy
Accessories:
1.3. Strap for attaching the urine collection bag, item number RKM1 – no more than 200 pieces per package;
2. Bedside medical urine collector (NC: 156370), consisting of:
2.1. Medical bedside urinal for adults (article number: MP209/1, MP201/1, MP202/1, MP209/2, MP201/2, MP202/2, MPR209/1, MPR01/1, MPR202/1, MPR209/2, MPR201/2, MPR202/2, MP101/1, MP101/2, MP102/1, MP102/2, MP109/1, MP109/2): with a tube length of 90 cm, 100 cm, 120 cm; volume of 1000 ml or 2000 ml; with or without a handle – no more than 200 pcs./pack.;
2.2. Instructions for use No. WS-004-IU – 1 copy
APPEARANCE AND GENERAL DESCRIPTION
A product consisting of a urine collection bag with a measuring scale, a tube with a conical connector, a urine backflow prevention valve, and a drain valve.
It has a T-shaped drain valve.
It may have a soft non-woven backing.
It has a mounting system.
The leg urine collector should have slots on the side surfaces of the urine collector.
The bedside urine collector should have holes or a handle for mounting.
The foot-mounted urine collector can be equipped with a belt for attaching the urine collector bag
Not subject to sterilization, disinfection, maintenance, or repair.
INDICATIONS FOR USE
Chronic or acute urinary retention;
• Incontinence or urinary incontinence;
• Collecting a urine sample for analysis;
• Performing a residual urine volume measurement and monitoring diuresis;
• Urinary excretion in patients in a coma;
• Urinary excretion in patients who are unable to empty their bladders on their own; patients who are under the influence of anesthetics or sedatives during the preoperative or rehabilitation period;
• Rehabilitation process for post-operative restoration of the genitourinary system.
The product is used under the prescription of a doctor. Depending on the indications, it is administered by a medical professional or by the patient themselves.
Contraindications
Individual intolerance to materials.
SIDE EFFECTS
When used, it may cause skin redness, rash, peeling, itching, and burning.
SCOPE OF APPLICATION
Departments of urology, internal medicine, surgery, oncology, obstetrics and gynecology, and therapy; rehabilitation; at home and in medical and preventive institutions.
POTENTIAL CONSUMER
Patients, patient relatives, care specialists, and medical professionals.
METHOD OF APPLICATION:
1. Open the package and remove the product.
2. In the urine collector with a removable connector, shorten the discharge tube to the required length.
3. If necessary, connect the adapter to the discharge tube and connect it to the catheter.
4. Make sure that the catheter and the tube are not twisted or bent, as this may prevent urine from flowing properly.
5. Use the holes and the handle on the top of the bag to secure the urine collector. / Use the slots on the side surfaces of the urinary catheter to secure the catheter using the straps (if available).
Technical specifications
a. Geometric dimensions of urinary bags
| Volume of the urinal, ml | Overall dimensions | |||
| Length, mm | Width, mm | Graduation step | ||
| 750 | 275 | 120 | 50 | |
| 1000 | 258 | 158 | 50 | 25(small graduation) |
| 2000 | 292 | 204 | 100 | |
b. Geometric dimensions of the discharge tube
| Overall dimensions | Value, mm |
| Inner diameter of the discharge tube | 4,8 |
| Outer diameter of the discharge tube | 6,2 |
| Diameter of the drain valve | 6,9 |
c. Geometric dimensions of the belt for attaching the urine collection bag
| Overall dimensions | Value, mm |
| Tape length, mm | 500 |
| Width of the tape, mm | 20 |
| Slot width, mm | 7 |
| Slot pitch, mm | 9 |
d. Geometric dimensions of the adapter for attaching the PKK1 catheter
| Overall dimensions | Value, mm |
| Length, mm | 38,2 |
| Range of external diameter, mm | 11,5 – 6,44 |
e. Geometric dimensions of the adapter for attaching the PKK2 catheter
| Overall dimensions | Value, mm |
| Length, mm | 32,4±0,25 |
| Range of external diameter, mm | 9,1 – 5,6 |
h. Mass characteristics
| Characteristic | Weight, g | ||
| Urinal | |||
| Tube length, cm | Volume, ml | ||
| 750 | 1000 | 2000 | |
| 50 | 33(±5) | – | – |
| 90 | 37(±5) | 39(±5) | 42(±5) |
| 100 | – | 42(±5) | 45(±5) |
| 120 | – | 45(±5) | 49(±5) |
| Accessories | |||
| Strap for attaching the urine collection bag | 8.5 | ||
| Adapter for catheter attachment | 1,2 | ||
PRODUCT MATERIALS
Type and duration of contact of a medical device according to GOST ISO 10993-1: Short-term contact medical devices that only contact the surface of intact skin.
| Name of the material | Manufacturer | Brand of the material | Purpose of the material | Model |
| The main composition of the product | ||||
| Polypropylene (PP) | Sibur LLC, Russia | PP G TU 2211-105-70353562-2014 | T-valve body | MN755/1, MN759/1, MN755H/1, MN759H/1, MN755H/2, MN759H/2, MN755H/2, MN759H/2, MN759H/2, MN755H/2, MNR755H/1, MNR759H/1, MNR755H/1, MNR759H/1, MNR755H/2, MNR755H/2, DR755N/2, DR759N/2, MP209/1, MP201/1, MP202/1, MP209/2, MP201/2, MP202/2, MPR209/1, MPR202/1, MPR202/1, MPR209/1, MPR209/2, MPR201/2, MP101/1, MP101/2, MP102/1, MP102/2, MP109 / 1, MP109 / 2 |
| Medical Polyvinyl chloride (PVC) | HONGKONG FONIX HITECH INDUSTRIES CO., LIMITED, China | PP H250 GP | Adapter for catheter attachment | |
| Lianqiao New Material Science&Technology Co.,LTD, China | SYPP-871-L | |||
| JIANGSU GRANSUN NEW MATERIAL CO., LTD, China | 6-006 | Film, Non-woven fabric, Tube | ||
| High-density polyethylene (HDPE) | HONGKONG FONIX HITECH INDUSTRIES CO., LIMITED, China | 1376-05 | Cap, valve core | |
| Guangdong Yaguangil Technology Co., LTD, China | 22-567A8 | |||
| High-pressure polyethylene (HDPE) | Sibur LLC, Russia | VIVILEN 60rPE 04404 FE TU 20.16.10-001-15724152-2021 | Cap, valve core | |
| Polyester | HONGKONG FONIX HITECH INDUSTRIES CO., LIMITED, China | PE500 | Strap for securing the urine bag | MNR755/1, MNR759/1, MNR755N/1, MNR759N/1, MNR755/2, MNR759/2, MNR755N/2, MNR759N/2 |
| Yiwu Keji Lugyage Accessories Co.Ltd, China | HGC-PE/17 | |||
| Granular Dye Concentrate for LDPE (HDPE) coloring (Blue) | GUANDONG RAINBOW DE PLASIC PIGMENT LIMITED BY SHARE CO., LIMITED, China | 9204B | The dye | MN755/1, MN759/1, MN755H/1, MN759H/1, MN755H/2, MN759H/2, MN755H/2, MN759H/2, MN759H/2, MN755H/2, MNR755H/1, MNR759H/1, MNR755H/1, MNR759H/1, MNR755H/2, MNR755H/2, DR755N/2, DR759N/2, MP209/1, MP201/1, MP202/1, MP209/2, MP201/2, MP202/2, MPR209/1, MPR202/1, MPR202/1, MPR209/1, MPR209/2, MPR201/2, MP101/1, MP101/2, MP102/1, MP102/2, MP109 / 1, MP109 / 2 |
| Lianqiao New Material Science&Technology Co.,LTD, China | RGB-005c | |||
| Cyclohexanone glue | ECOS-1 JSC, Russia | TU-2633-012-44493179-98 | Glue | |
| Granular Dye Concentrate for LDPE (HDPE) coloring (Green) | GUANDONG RAINBOW DE PLASIC PIGMENT LIMITED BY SHARE CO., LIMITED, China | 9202G | The dye | MN755/1, MN759/1, MN755H/1, MN759H/1, MN755H/2, MN759H/2, MN755H/2, MN759H/2, MN759H/2, MN755H/2, MNR755H/1, MNR759H/1, MNR755H/1, MNR759H/1, MNR755H/2, MNR755H/2, DR755N/2, DR759N/2, MP209/1, MP201/1, MP202/1, MP209/2, MP201/2, MP202/2, MPR209/1, MPR202/1, MPR202/1, MPR209/1, MPR209 / 2, MPR201 / 2, MPR209 / 2, MPR209 / 2 |
| Lianqiao New Material Science&Technology Co.,LTD, China | RGB-004d | |||
| Solvent ink for printing on PVC film (Green) | Triada LLC, Russia | КС-З-11263 | The dye | |
| Solvent ink for printing on PVC film (Blue) | Triada LLC, Russia | КС-С-23644 | The dye | MN755/1, MN759/1, MN755H/1, MN759H/1, MN755H/2, MN759H/2, MN755H/2, MN759H/2, MN759H/2, MN755H/2, MNR755H/1, MNR759H/1, MNR755H/1, MNR759H/1, MNR755H/2, MNR755H/2, DR755N/2, DR759N/2, MP209/1, MP201/1, MP202/1, MP209/2, MP201/2, MP202/2, MPR209/1, MPR202/1, MPR202/1, MPR209/1, MPR209/2, MPR201/2, MP101/1, MP101/2, MP102/1, MP102/2, MP109 / 1, MP109 / 2 |
CONDITIONS OF TRANSPORTATION, STORAGE, AND OPERATION
– temperature: from 0 to +40 ºC;
– humidity: No more than 80%.
The product should be protected from direct sunlight, and should not be exposed to heat or freezing. Store in a dry, clean place.
Keep out of reach of children.
DISPOSAL OF A MEDICAL PRODUCT
At home:
after use, dispose of it with solid household waste.
In medical and preventive institutions:
Unused products and packaging are disposed of in accordance with local regulations as Class A waste in accordance with SanPiN 2.1.3684-21.
When using medical products in infectious disease departments, used products are disposed of in accordance with local regulations as Class B waste in accordance with SanPiN 2.1.3684-21.
Expiration date
Product shelf life: 5 years from the date of manufacture indicated on the packaging.
Do not use the product after the expiration date.
MANUFACTURER'S GUARANTEES
The manufacturer guarantees that the product meets the declared characteristics if the consumer follows the conditions of use, transportation, and storage.
The product is guaranteed to remain sterile for 5 years from the date of manufacture indicated on the packaging.
INFORMATION ABOUT COMPLAINTS
Claims are made in accordance with the established procedure at the manufacturer's address
APRIL LLC, Russia
LIST OF STANDARDS
GOST ISO 10993-1 Medical devices. Assessment of the biological effect of medical devices. Part 1. Assessment and research in the risk management process
SanPiN 2.1.3684-21 Sanitary and epidemiological requirements for the maintenance of urban and rural settlements, water bodies, drinking water and drinking water supply, atmospheric air, soils, residential premises, operation of production and public premises, organization and implementation of sanitary and anti-epidemic (preventive) measures
Our contacts
Address of the trading house:
Krasnodar Territory, Krasnodar, Morskaya Street, 15
Production address:




